News and updates
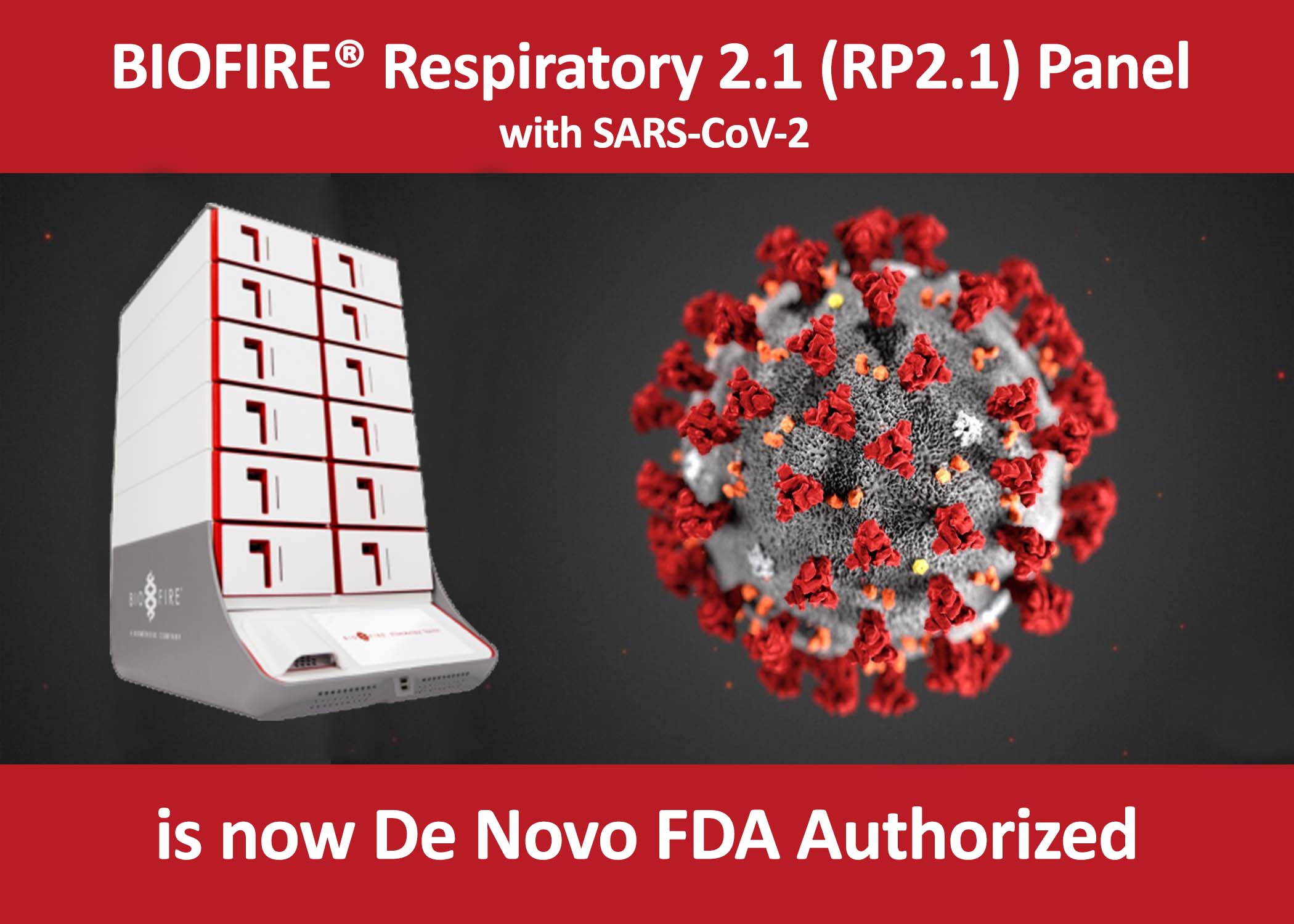
BIOFIRE Respiratory 2.1 - RP2.1 Panel with SARS-CoV-2 obtains De Novo FDA Authorization
On March 18, 2021 – bioMérieux, a world leader in the field of in-vitro diagnostics, announced that BioFire Diagnostics, its subsidiary specialized in molecular syndromic infectious disease testing, has received U.S. Food and Drug Administration (FDA) De Novo authorization for the BIOFIRE® RP2.1 Panel.
This panel allows for the detection of 22 viral and bacterial pathogens responsible for respiratory infections, including SARS-CoV-2 (the cause of COVID-19 disease). The panel is the first SARS-CoV-2 diagnostic test of any kind that has been granted De Novo status by the U.S. FDA, having gone through the normal U.S. FDA review pathway outside of the Emergency Use Authorization (EUA) track.
The BIOFIRE® RP2.1 Panel allows healthcare providers to quickly identify common respiratory pathogens found in patients presenting with acute respiratory tract infection, using one simple test. The BIOFIRE® RP2.1 Panel yields result in approximately 45 minutes using nasopharyngeal swab (NPS) samples in transport media or saline. It runs on the fully automated BIOFIRE® FILMARRAY® 2.0 and BIOFIRE® Torch Systems with only 2 minutes of sample preparation time.