News and updates
RP 2.1 with SARS-CoV-2 obtains FDA Emergency Use Authorization
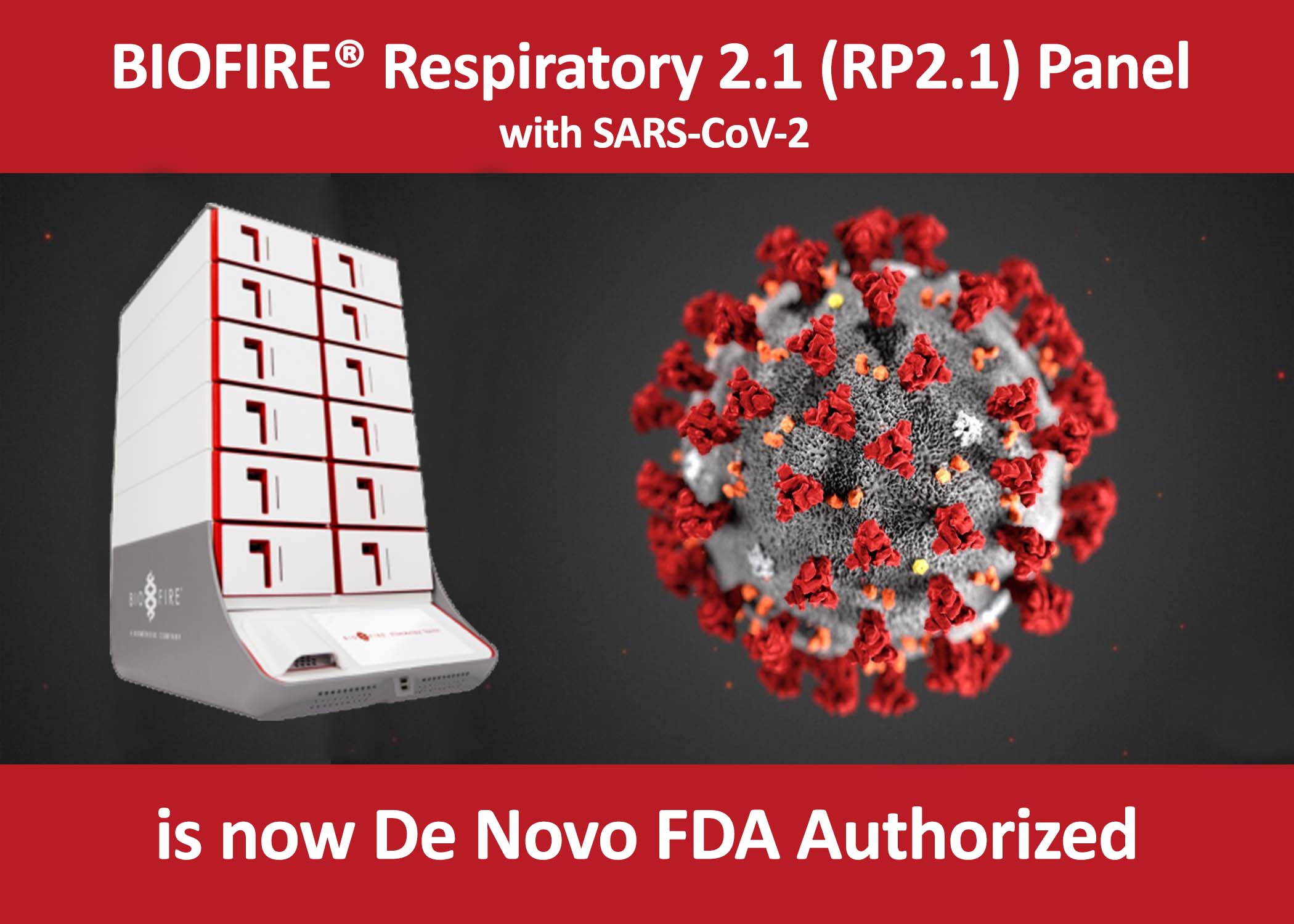
BIOFIRE® Respiratory Panel 2.1 (RP2.1) with SARS-CoV-2 obtains FDA Emergency Use Authorization
BioMérieux, a world leader in the field of In-Vitro Diagnostics, on May 4, 2020, announced that BioFire Diagnostics has received Emergency Use Authorization by the U.S. Food and Drug Administration for the BIOFIRE® RP2.1 panel.
• The inclusion of SARS-CoV-2 in the BIOFIRE® RP2.1 panel allows healthcare providers to quickly identify patients with common respiratory pathogens, as well as those with COVID-19, using one simple test.
• The BIOFIRE® RP2.1 panel takes approximately 45 minutes.
• It runs on the fully automated FILMARRAY® 2.0 and FILMARRAY® TORCH systems and is extremely easy to use.
Outside of the USA, bioMérieux is simultaneously pursuing CE Mark certification for the BIOFIRE® Respiratory 2.1plus (RP2.1plus) panel, which also includes detection of MERS-CoV, on an accelerated timeline.