News and updates
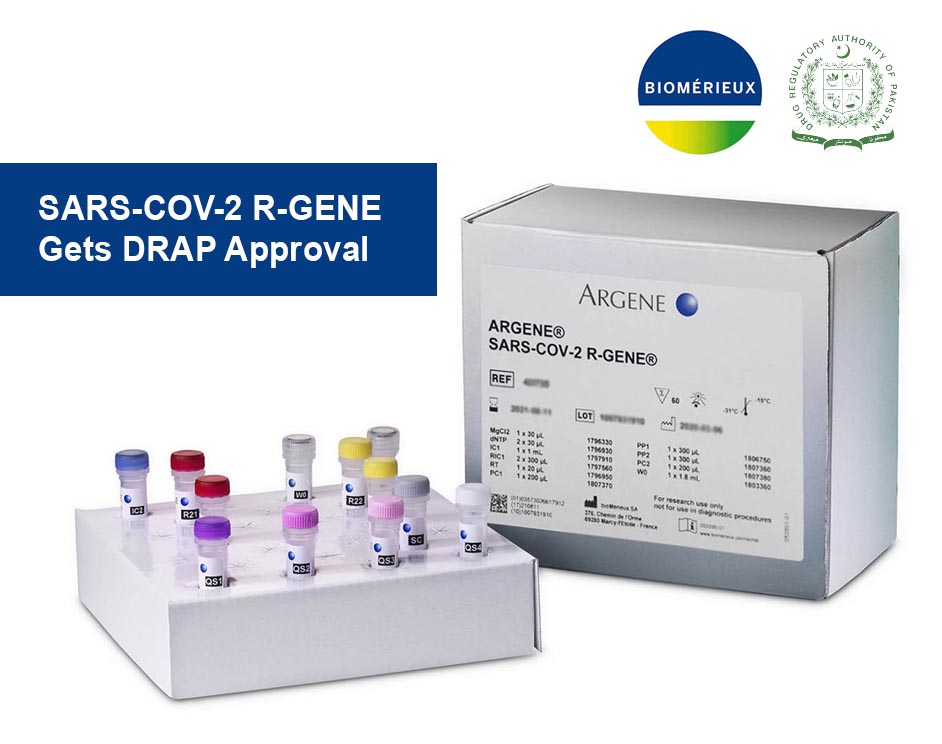
Biomerieux SARS-COV-2 R-GENE Gets DRAP Approval
Global Marketing Services is proud to announce that we have been awarded the registration certificate from Drug Regulatory Authority Pakistan (DRAP) for the Biomerieux SARS-COV-2 R-GENE (Real-time Amplification and Detection Kit.
The SARS-COV-2 R-GENE® test is an open assay, meaning that it may be performed by any laboratory using PCR technology on most commercially-available nucleic acid extraction and amplification platforms. Results are delivered in 4 to 5 hours, and a large number of patient samples may be processed simultaneously.
GMS also provides complete solutions for the Testing of COVID-19. For more information related to GMS COVID-19 SOLUTIONS, you can check our website.