News and updates
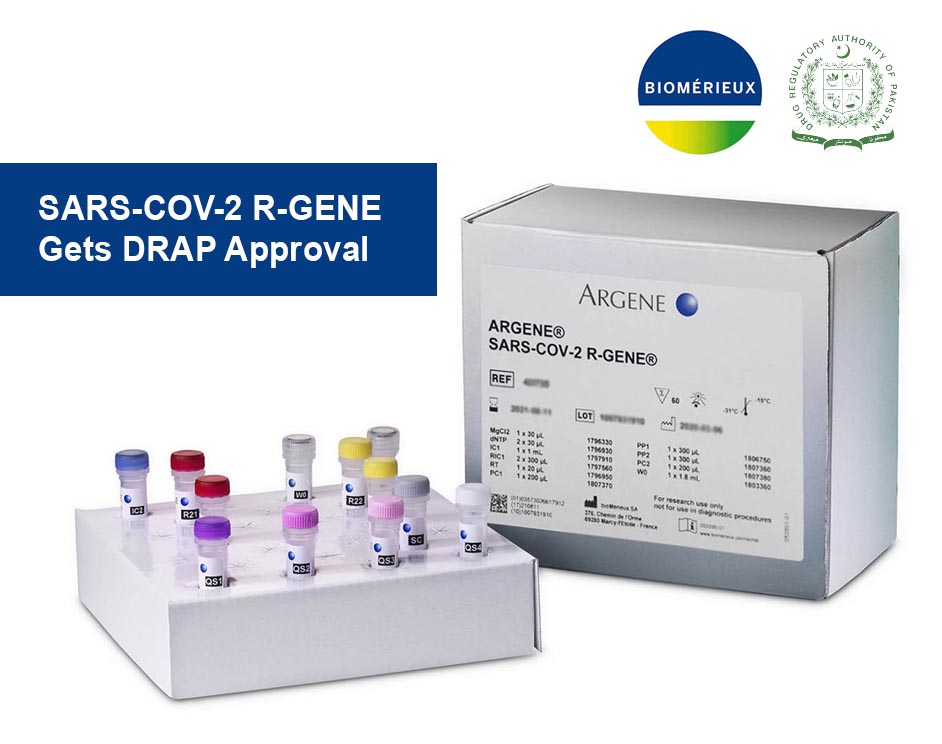
BioMérieux SARS-COV-2 R-GENE obtains FDA Emergency Use Authorization
BioMérieux, a world leader in the field of In-Vitro Diagnostics, on May 6, 2020, announced that SARS-COV-2 R-GENE has received Emergency Use Authorization by the U.S. Food and Drug Administration.
SARS-COV-2 R-GENE is a real-time qualitative kit for the detection of SARS-Cov-2 form Nasopharyngeal swabs. The kit design consists of 2 triplex PCR designs…PCR 1 for detection of N -gene, Rdrp gene, and PCR 2 for detection of E gene with 120 number of tests.
It takes almost 90 minutes after the extraction process to give final results and the kit is validated on many extraction and amplification platforms.