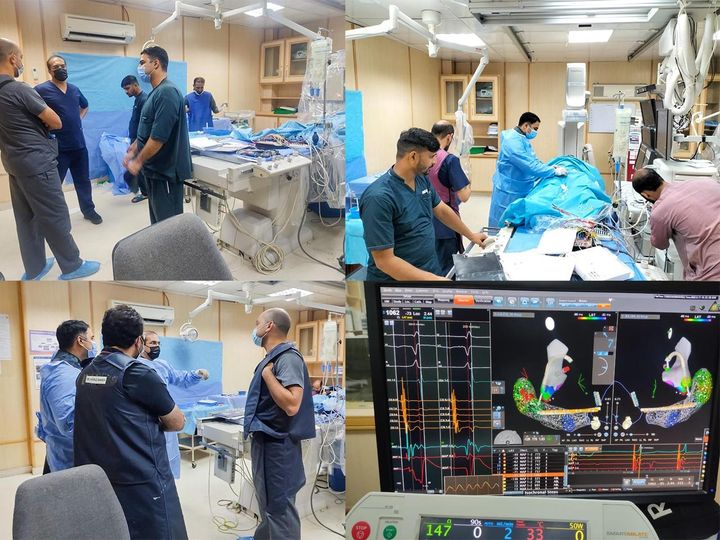
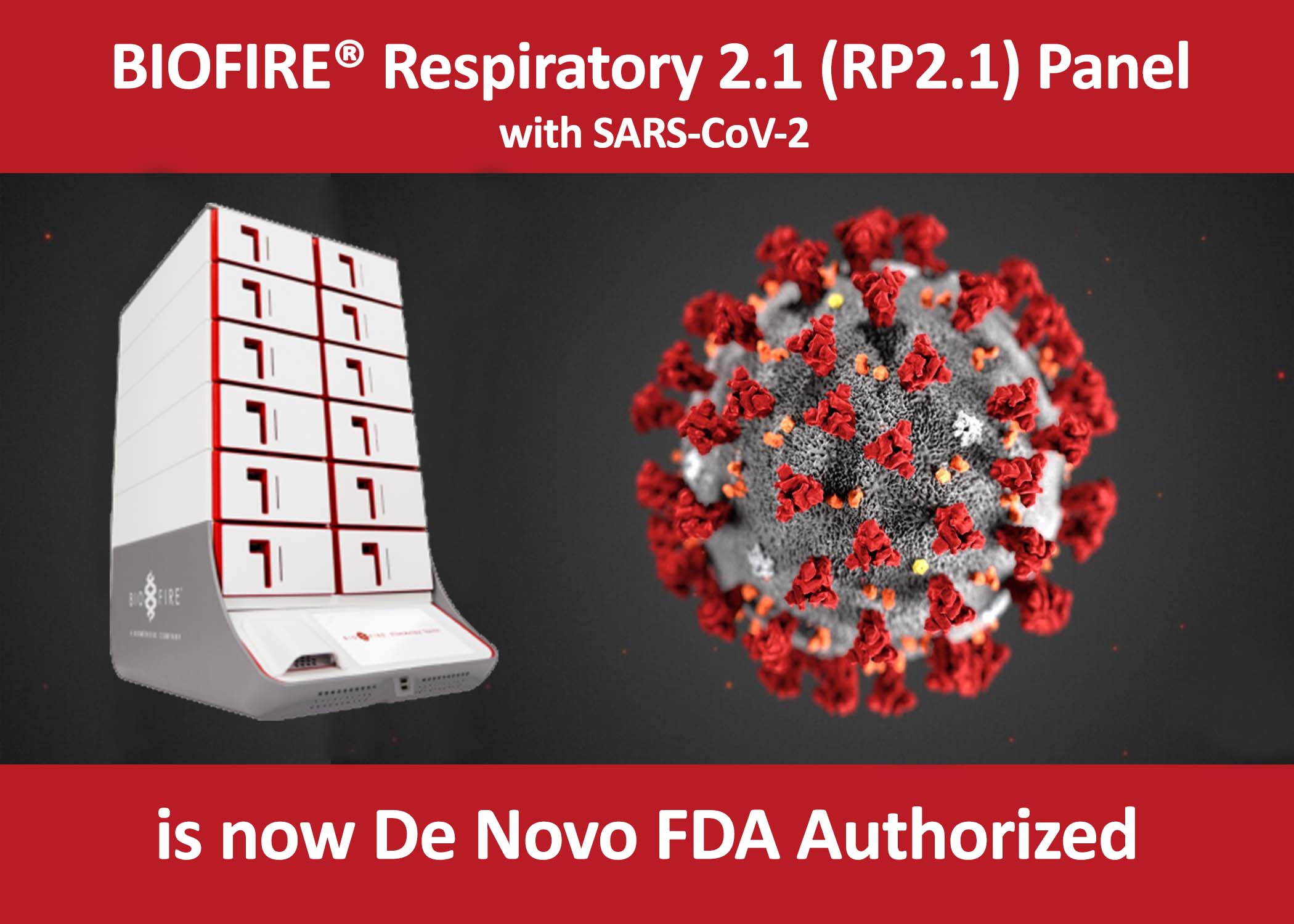
News and updates
Xpert® HIV Viral Load has been accepted for the WHO list of prequalified IVD
Xpert® HIV-1 Qual Assay with GeneXpert® Dx, GeneXpert® Infinity-48s, and GeneXpert® Infinity-80 Infinity-80 manufactured by Cepheid AB, CE marked regulatory version, was accepted for the WHO list of prequalified in vitro diagnostics and was listed on 13 June 2016
Xpert® HIV-1 Qual Assay, performed on the GeneXpert® Instrument Systems, is a qualitative in vitro diagnostic test designed to detect human immunodeficiency virus Type 1 (HIV-1) total nucleic acids using human venous whole blood and capillary or EDTA venous dried blood spots from individuals suspected of HIV-1 infection. It is intended to aid in the diagnosis of HIV-1 infection in conjunction with clinical presentation and other laboratory markers